Researchers in the Vanderbilt University School of Medicine have received three grants totaling $13.6 million from the National Institutes of Health to develop molecular “atlases” of the brain, kidney, eye and other tissues.
Their goal is to find new and better ways to diagnose, treat and prevent a host of human ailments, from kidney failure to dementia.
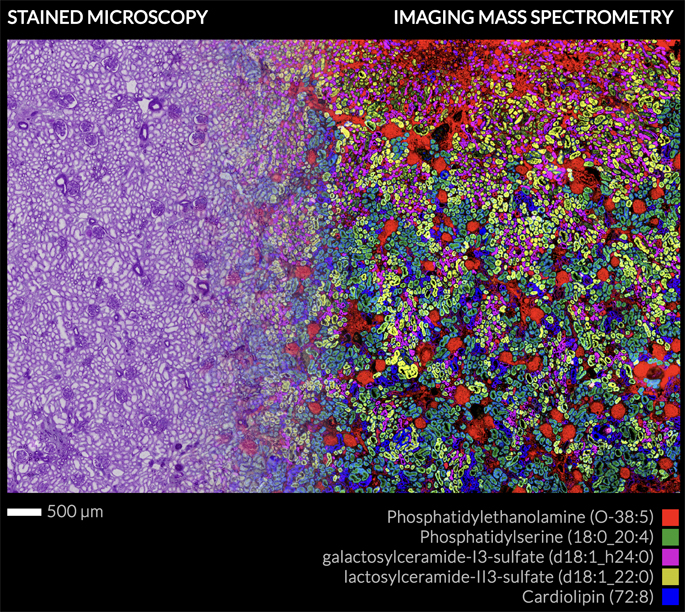
The grants will utilize imaging technologies developed at the Vanderbilt Biomolecular Multimodal Imaging Center (BIOMIC) and Mass Spectrometry Research Center that are enabling construction of three-dimensional, and minutely detailed descriptions of human tissue at the molecular level.
“We are bringing the best molecular imaging tools together with advanced machine learning approaches to create a fully integrated set of technologies that provide a systems biology view of tissue at cellular resolution,” said BIOMIC director Jeff Spraggins, PhD, a principal investigator of the new grants.
The grants include:
- A four-year, $7.1 million renewal grant from the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) and the NIH Common Fund to continue development of a molecular atlas of the human kidney that began in collaboration with co-principal investigator Richard Caprioli, PhD, in 2018.
- A companion five-year, $3 million grant from NIDDK that will establish a Kidney Precision Medicine Project Tissue Interrogation Site at Vanderbilt to analyze patient biopsies with the goal of determining the molecular drivers of acute kidney injury and chronic kidney disease.
- A five-year, $3.5 million grant from the National Institute on Aging entitled “Elucidating Molecular Drivers of Aging and Alzheimer’s Disease via Multimodal Imaging Mass Spectrometry.”
Caprioli, director of the Vanderbilt Mass Spectrometry Research Center, is Stanford Moore Professor of Biochemistry, and professor of Medicine, Pharmacology, and Chemistry. In the late 1990s, he and his colleagues pioneered the use of laser-based mass spectrometry to produce images of lipids, proteins and other molecules in cells and tissues.
The technology, called matrix-assisted laser desorption/ionization imaging mass spectrometry, or MALDI IMS, is essentially a “molecular microscope.” By detecting and measuring changes in the spatial arrangement, distribution and expression levels of different molecules, MALDI IMS can produce an extraordinarily detailed molecular portrait of disease.
In 2011 the National Center for Research Resources awarded Vanderbilt University a five-year, $10.3 million grant to establish a National Research Resource for Imaging Mass Spectrometry. That support was renewed for another five years in 2016 with a second, $10.5 million grant.
Spraggins, assistant professor of Cell & Developmental Biology, Biochemistry, and Chemistry, joined the Vanderbilt faculty in 2012.
In 2015, the Vanderbilt researchers achieved the first “image fusion” of mass spectrometry and microscopy — a technological tour de force that allowed them to see the molecular makeup of tissues in high resolution.
In 2018 Caprioli and Spraggins established BIOMIC as a tissue-mapping center in the national Human Biomolecular Atlas Program (HuBMAP). Its first challenge: create a high-resolution, three-dimensional atlas of the human kidney to better understand what goes wrong in end-stage renal disease, one of the nation’s most debilitating and expensive medical conditions.
Supported by the NIH Common Fund, HuBMAP is a consortium of 350 collaborators from 62 institutions who are developing next-generation molecular analysis technologies and computational tools that will enable them to construct an atlas of the function and relationships among cells in every tissue in the human body.
Vanderbilt’s renewal grant from the NIDDK and NIH Common Fund will enable BIOMIC to continue “fusing” the mass spectrometry-based imaging capabilities of the Mass Spectrometry Research Center and National Research Resource for Imaging Mass Spectrometry with other state-of-the-art technologies under development at the university and VUMC.
These technologies include highly multiplexed immunofluorescence microscopy, autofluorescence microscopy, spatial transcriptomics and proteomics, and single-cell RNA-sequencing.
BIOMIC also leverages the advanced biocomputational infrastructure available through the Vanderbilt’s Data Analysis Core laboratories, and in collaboration with Raf Van de Plas, PhD, and Joana Gonçalves, PhD, at Delft University of Technology in the Netherlands.
A companion grant from NIDDK supports creation of a Kidney Precision Medicine Project Tissue Interrogation Site.
By integrating and mining molecular imaging and spatial optics technologies, Spraggins said, “we aim to discover molecular markers of disease in specific cell types in the kidney and to identify potential biomarkers and optimal points of therapeutic intervention.”
The third grant, from the National Institute on Aging, will use imaging mass spectrometry in tandem with various forms of microscopy, multi-omic spatial profiling, and machine learning to map cellular and molecular alterations associated with normal aging and Alzheimer’s disease in brain tissue.
Co-principal investigators are Matthew Schrag, MD, PhD, assistant professor of Neurology and director of the Cerebral Amyloid Angiopathy Clinic at VUMC, and Renã A. S. Robinson, PhD, professor of Chemistry and Dorothy J. Wingfield Phillips Chancellor’s Faculty Fellow.
Schrag is investigating how cerebral amyloid angiopathy, the accumulation of amyloid beta-peptide deposits in small- to medium-sized blood vessels of the brain and leptomeninges, the membranes that surround the brain and spinal cord, contributes to cognitive impairment in Alzheimer’s disease.
Robinson uses novel proteomics and other ‘omics approaches to analyze human tissues and animal models with the goal of understanding the molecular basis of health disparities in Alzheimer’s disease, and how the heart, kidney, and other peripheral organs, as well as immune T-cells, may contribute to it.
“We believe that our spatially resolved and molecularly comprehensive approach will lead to improved mechanistic understanding of Alzheimer’s disease, and that these insights could inform better treatment options,” Spraggins said.
Added Caprioli: “The basic technologies and protocols developed as a result of these grants, and those developed by our colleagues, are translatable to the molecular discovery of any disease process and hold great potential for direct clinical use including bedside care.”












